Abstract
Background Initial tyrosine kinase inhibitor (TKI) therapy strategies in persons with newly-diagnosed chronic phase chronic myeloid leukaemia (CML) include the standard dose imatinib, a 2 nd generation TKI, staring with imatinib and then increasing the dose or switching to a 2 nd generation TKI if the 2020 European LeukemiaNet (ELN) response landmarks are not met. Which therapy strategy is best is still uncertain. There is no predictive score for failure-free survival in this setting to help physicians choose between these alternatives.
Objective Develop and validate a predictive score for failure-free survival (FFS; FFS predictive score [FFSPS]) in persons with newly-diagnosed chronic phase CML receiving initial imatinib therapy.
Methods Data from 1321 consecutive subjects receiving initial therapy with imatinib were interrogated to identify co-variates predicting FFS with failure defined according to the 2020 ELN criteria. Cut-offs for continuous variables were determined by analyzing receiver-operator characteristic (ROC) curves, and a Cox multi-variable regression model was used to identify predictive co-variates in a training dataset (n = 880). A weighted predictive score (FFSPS) was developed and validated in 441 other subjects.
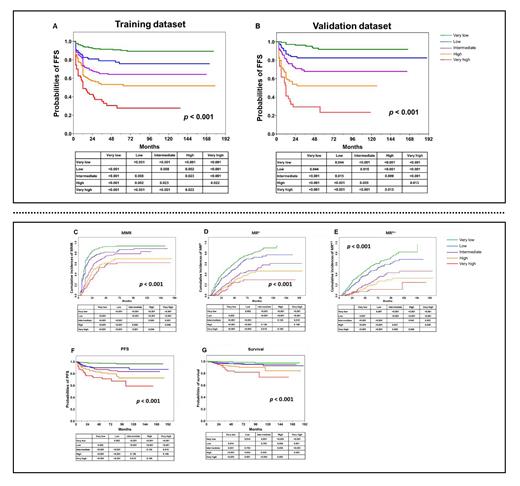
Results In the training dataset, WBC ≥ 120 × 10E+9 /L (Hazard Ratio [HR] = 1.7 [95% Confidence Interval (CI), 1.1, 2.2]), haemoglobin concentration ≤ 113.5 g/L (HR = 2.3 [1.7, 3.1]), blood basophils ≥ 11.5% (HR =1.6 [1.0, 2.3]) and EUTOS long-term survival (ELTS) risk score (intermediate-risk, HR = 1.9 [1.4, 2.6]; high-risk, HR = 3.7 [2.2, 5.0]) were significantly-associated with worse FFS and each assigned 1 point in the scoring system except ELTS high-risk which was assigned 2 points. According to the FFSPS, the training dataset were classified into 5 subgroups: the very low- (score = 0; n = 307, 35%), low- (score = 1; n = 176, 20%), intermediate- (score = 2; n = 183, 21%); high- (score = 3; n = 152, 17%) and the very high- (score ≥ 4; n = 62, 7%) risk subgroups. There were significant differences in the 7-year probabilities of FFS among the 5 subgroups (89% [84, 92%] vs. 76% [67, 83%] vs.64% [56, 71%] vs. 52% [43, 60%] vs.28% [17, 40%], p < 0.001, Figure A). The concordance statistic (C-statistic) based on the FFSPS was 0.75 (0.69, 0.78) in the training dataset. And in the validation dataset, 155 (35%), 88 (20%), 94 (21%), 70 (16%) and 34 (8%) subjects were classified as the very low-, low-, intermediate-, high-, and the very high-risk subgroups, among which there were significant differences in the probabilities of 7-year FFS (p < 0.001, Figure B). The C-statistic in the validation dataset was 0.74 (0.67, 0.80). Moreover, the FFSPS was also correlated with probabilities of major molecular response (MMR), MR 4.0 and MR 4.5, progression-free survival (PFS) and survival (all p-values < 0.001, Figure C-G).
Conclusions We describe a predictive score for FFS in persons with newly-diagnosed chronic phase CML receiving initial imatinib therapy. The score identifies persons with different probabilities of success. These data should help physicians estimate the best TKI-therapy strategy in this setting.
No relevant conflicts of interest to declare.